Eutrophication: the monster hiding in plain sight
Vicky Ellis examines the issue of nutrient neutrality and says it is high time we tackled the continuing abuse of our waterbodies
London was once a matrix of towns and villages with beautiful and picturesque meandering rivers fed by freshwater springs and tributaries. It’s hard to visualise now but, as the city sprawled, the rivers that once teemed with life were used as dumping grounds during the 1800s. They became desperately polluted – like open sewers or disease pits – and had an overpowering stench.
Eventually, about 12 of these rivers were entombed and incorporated into the sewer network of London, never to flow above ground again, with only the odd stink pipe here and there left to tell of their demise.
You would think this sad legacy of pollution and destruction of our natural waterways would be behind us and that we would have learnt from our filthy past – but it would seem not.
We have all heard the word ‘eutrophication’ used lately, but what does it mean and why are the associated nitrogen and phosphate so damaging for our waterbodies?
According to Myriam Webster, “the definition of eutrophication is the process by which a body of water becomes enriched in dissolved nutrients (such as phosphates) that stimulate the growth of aquatic plant life usually resulting in the depletion of dissolved oxygen”.
To date, an estimated potential development of 120,000 new homes and 74 local authorities (LAs) with protected sites (Special Protection Areas, Special Areas of Conservation and Ramsar sites), including some in Kent, have been affected by human-induced eutrophication pollution.
These LAs were told by Natural England (NE) that any proposed change of land use or development in ‘catchment’ areas around these protected sites with waterways must not proceed without a Habitats Regulations Assessment (HRA) to determine any potential adverse effects on protected sites.
However, to enable the moratorium on building to be lifted, where developments are likely to fail the HRA requirements, the developers will be requested to take mitigation action by either building additional mitigation plans on-site, working with the LAs to place mitigation off-site (wherever that might be), or buy nutrient credits via a trading scheme, ie where other landowners within the catchment area have taken action to reduce their nutrient load.
How this will be policed, ie how these landowners who have reduced their nutrient load will be monitored for continuous reduction, is not clear.
In this article we examine why both nitrogen and phosphate cause so much harm and damage to aquatic ecosystems, to the economy and to public health. To understand why eutrophication is so damaging, one needs to understand the vital role they play in plant health.
Understanding the role nitrogen and phosphate play in plant health
Nitrogen (N) is a macronutrient (part of the chlorophyll molecule giving plants their green colour). It is essential for plant function and a vital component of amino acids – the building blocks of plant protoplasm, a translucent substance integral to the structural fabric of the plant and that is essentially the living matter within the cells. Protoplasm plays a role in flower differentiation, growth, health and quality of fruit.
These macronutrients enable plants to utilise sunlight via photosynthesis and thus aid plant growth.
Phosphorus (P) is important in cell division and the production of new tissue, along with complex energy transformations within the plant, aiding the conversion of other nutrients to usable building blocks enabling growth. Phosphorus is a component of DNA containing genetic data for all living organisms. It is also part of the RNA reading the DNA’s genetic coding, responsible for the synthesis of proteins that form the structure of aquatic plants. DNA and RNA are linked by phosphorus.
Adenosine triphosphate (ATP) is the source of energy for use at cellular level. ATP’s structure is a nucleoside triphosphate, made up of three serially bonded phosphate groups, a ribose sugar and a nitrogenous base called adenine.
P is contained in many minerals within our soils and N makes up 80 per cent of gases present in our atmosphere, but they do not exist in a form that can be readily utilised by plants or animals.
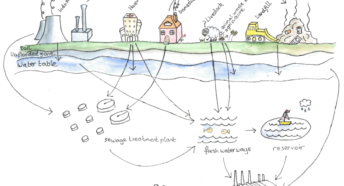
Both N and P are crucial to all living things being able to power their cells. It all goes wrong when human activity interferes with this delicate balance and then N and P just become pollutants leaching into waterbodies both above ground and below (aquifers), or via effluent being released directly into the rivers and seas. Even rainwater now contains pollutants.
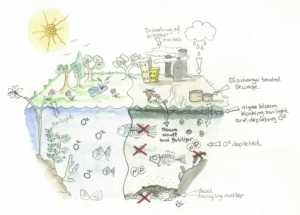
Once pollution has occurred, N and P feed the algae in the water, which causes it to bloom and proliferate abundantly, choking the water and robbing the aquatic life below of sunlight, leading to aquatic dead zones, slowing the river flow and in some cases releasing toxins into the water.
Eventually the algal bloom will die back and sink to the bottom, where microbes get to work breaking down this organic matter. The dead and rotting algae feed the microbes and they begin to proliferate, using up the available dissolved oxygen required for respiration by animals and plants.
Once the oxygen levels reach hypoxic or anoxic levels, other aquatic biota in the water begin to suffocate and die and, in turn, also feed into the dead and decomposing organic matter, accelerating oxygen depletion yet further. At this stage the water has reached the point where it can no longer cleanse itself.
Once the human-induced pollution has reached this level, the knock-on effect is a complete imbalance of the aquatic ecosystem. The delicate food web has effectively broken down and the food organisms that have died are not available for fish, birds and mammals to feed on, leading to a localised population crash and an ecological imbalance.
The high rate of photosynthesis that occurs with eutrophication can also be detrimental due to the depletion of dissolved inorganic carbon, raising pH levels to such a high during the day that they effectively inhibit chemical cues that some organisms rely on for their survival by impairing their chemosensory abilities.
Algae are as much a part of the ecosystem of waterbodies as any other aquatic organism, but we only notice them when the natural balance of nature is disrupted and they bloom out of control.
In certain extreme cases, anaerobic conditions lead to the proliferation of bacteria that produce toxins that are fatal to aquatic life and other higher organisms.
One such infamous group of algae are cyanobacteria, photosynthetic prokaryotes (so not true algae but bacteria) more commonly referred to as blue-green algae. Cyanobacteria can produce the toxin microcystin and anatoxin-a, which is what makes us and our pets ill if ingested and is a danger to livestock and wildlife.
Blue-green algae produce beta-methylamino-I-alanine (BMAA), an amino acid compound that may have causal links to neuron diseases similar in nature to Alzheimer’s in humans with prolonged exposure.
These microscopic organisms are naturally present in lakes and streams and can either be unicellular, filamentous or colony-forming species appearing as scum on the surface but can also be present at depths of two to nine metres, therefore not necessarily visible from the surface.
Due to their ability to utilise low levels of nitrogen and phosphorous, they can out-compete other algae, despite generally growing more slowly. Blue-green algae tend to bloom on sunny, still or stagnant, warm water that is nutrient-rich. Not all blue-green algae form toxins, but it is very difficult to tell without testing the water which species are involved. Algae of the genus anabaena are generally the ones involved in poisonings, but they are not the only toxic blue-green algae.
Poisoning by cyanobacteria has been documented globally as a threat as far back as 1878. However, the phenomenon of blue-green algal blooms has become more frequent over time; this is due to both increased eutrophication pollution and also climate change, the two key factors cyanobacteria loves – nutrients and heat, the optimal temperature being 15°C-30°C, with an optimal pH of 6-9.

Harmful algal blooms (HABs) not only pose a threat to public health but also to drinking-water systems, aquaculture, commercial fishing and fisheries, recreational fishing and, ironically, livestock farming.
Eutrophication for the nitrogen load generally entails diffused sources primarily from agricultural land.
Other sources of nitrogen include gases such as ammonium from spreading manure, nitrogen oxides from ships – transmitted via the atmosphere to oceans via precipitation – aquaculture, wastewater treatment plants, industrial water and contaminated oceans. Phosphate generally comes from domestic and industrial sewage, wastewater and run-off and leaching from land treated with fertilisers, including muck-spreading.
The effects of human-induced eutrophication can be profound, not only from an ecological stance but also an economic perspective. Even if we only consider the ecological impacts, the devastation and fundamental change in the ecological balance and the inability of the waterbodies to cleanse themselves are likely to have an acute effect on our future health.
Not only are we at risk of losing some of our most valued and precious protected open spaces, we are also at real risk of losing our own health and well-being as a direct result if we carry on ignoring the damage we are inflicting on the natural world.
This is why NE has issued new nutrient-neutrality rules. Housing Today’s campaign A Fair Deal for Housing addresses the concerns of developers caught up in NE nutrient-neutrality rules and the hundreds of thousands of pounds they are losing.
However, this pollution problem is much bigger, even in terms of economics – what is really needed is a fair deal for all of us and for nature. It is simply not sustainable to keep pumping more and more sewage, treated or untreated, into our seas, rivers and streams from more and more new developments without some kind of effective mitigation in place.
We have a responsibility and duty of care to the environment that, in turn, protects and provides for us.
The threat of eutrophication affects us all, not just directly but indirectly, too, because it is not simply about a few aquatic organisms dying on a protected site somewhere – if our delicate ecological system collapses, we collapse along with it.